A water drop on a glass surface |
This photograph is, however, representative of a phenomenon that exists in both Chemistry and Biology called Adsorption.
Adsorption is the physical adherence of a material (the adsorbate, in either gas or liquid or solid form) onto a surface of another material (the adsorbent)usually by "bonding" of ions, atoms, molecules or biomolecules between the two materials.
 |
| Adsorbate Molecules on Adsorbent Surface |
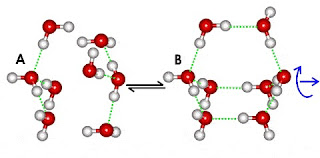
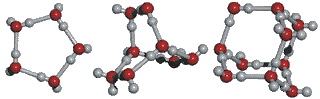
Water Clustering
A unique property of water, due its unique physical molecules, is that water molecules cluster in nearly an infinite number of shapes, two of which are shown in the following illustration:
This ability to cluster and form new chemical forms gives water many of its unique properties. Desiccants (like silica gel) use adsorption to remove water from air.
Adsorption in Biology
In the Science of Virology, the adhesion of a substance to an organic particle, e.g. the adhesion of a virus to a cell, is also known as Adsorption.
In Chemistry the adhesion is fueled by chemical bonds (e.g. by water's hydrogen bonds) while in Virology, viruses adsorb to their host cell surface via specific anti-receptor molecules, often glycoproteins. One example of glycoproteins found in the human body is mucins, which are secreted in the mucus of the respiratory and digestive tracts, where sugars attach to the mucins and give then considerable water-holding capacity.
It still remains to be seen if glycoproteins, and its brethren,can be used to extract water from the air in considerable volume!
Photo Sources:
http://en.wikipedia.org/wiki/Surface_energy
http://en.wikipedia.org/wiki/Adsorption
http://www.lsbu.ac.uk/water/abstrct.html#min

No comments:
Post a Comment